Clinicaltrials.gov is a central database of clinical trials for all conditions, including ALS. It is an important resource for finding a clinical trial that is right for you. All trials enrolling subjects in the United States, and most trials being conducted worldwide, are listed on this site, making it a great place to start your search.

Here are some helpful tips for searching the clinical trial databse.
- Click on this link www.clinicaltrials.gov
- Once on the site, you can enter as many or as few search terms as you wish.
- You may want to do a very broad search to get started, using “Amyotrophic Lateral Sclerosis” as your only search term. This will bring up the largest number of trials. Tip: Use “Amyotrophic Lateral Sclerosis” rather than ALS to avoid bringing up non-ALS trials.
- You can narrow down your search results by selecting "Recruiting and not yet recruiting studies" within the "United States". - Click Search or refine your search further by clicking Advanced Search
- The Advanced Search allows several ways to refine your search. Here you can specify many different types of restrictions, including trials that are currently recruiting participants, observational or interventional trials, location, and other criteria.
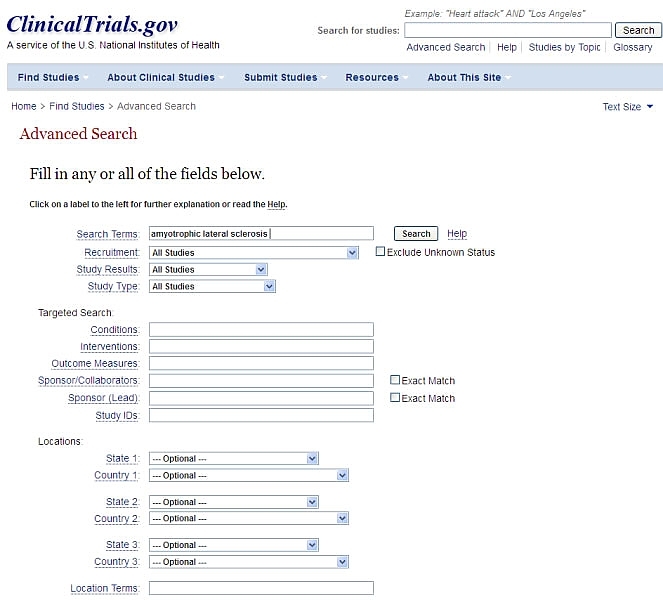
From this page, when you have clicked "search" you will see the "On a Map" tab. Clicking this will show the location of the studies you found on a map.
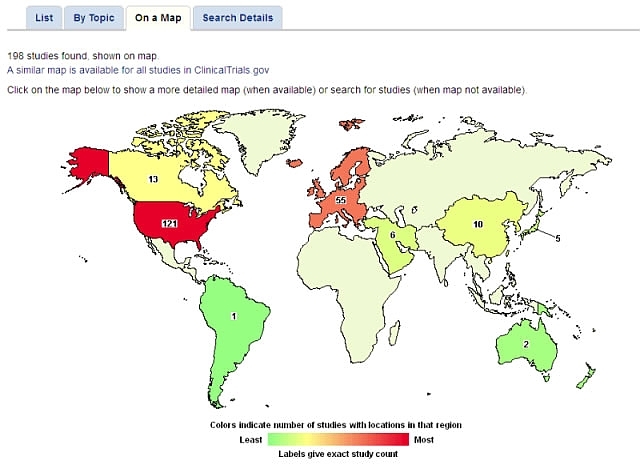
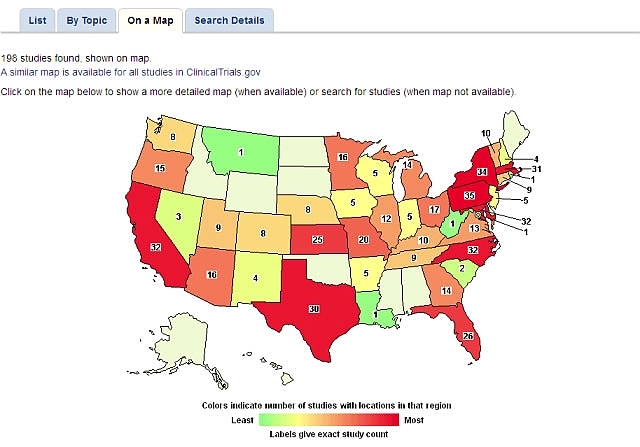
You can click on any region on the world map to find more details. For instance, if you click on the United States, a new map showing state-by-state results appears. You can then click on an individual state to bring up study details.
Example:
Let’s say you want to find a trial of a new treatment that is currently recruiting patients in your state (Florida, for instance). That means you want an Interventional (testing a new treatment) trial, currently Recruiting patients in Florida. Your search screen should look like this:
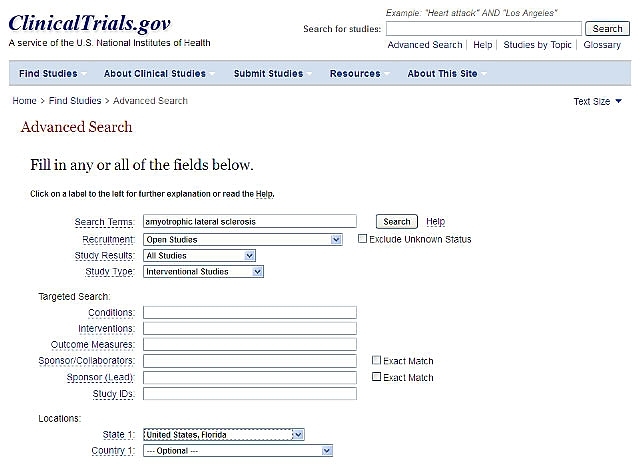
Press “Search” to find trials that meet that condition.
Understanding Your Search Results
Your results page lists the trials meeting your search criteria. You can click on each to get further details about the trial. The details may be challenging to understand at first glance. Here are some tips to help you get the most from this information:
- The best way to begin may be to read the “Detailed Description” about halfway down the page. This provides a discussion of the rationale and design of the trial.
- Look just below that at the “Eligibility” information. If you meet the inclusion criteria, and are not excluded by the exclusion criteria, you are likely to be eligible for the trial.
- You can also find information on the page about the primary and secondary “outcome measures." These are the measurements the researchers will be conducting in the trial.
- The study location closest to you may be listed on the page, or you may need to click the link “Show Study Locations” to bring up a list of all the locations.
Contacting the Study Team
Although clinicaltrials.gov is an excellent resource and provides accurate information, important aspects of a trial may not be made clear on the site. For this reason, if you are interested in a study, it is a good idea to discuss it with your ALS physician.
A good next step is to call the study team at the number listed on the website. Each study site will list the name and location of the institution, the name of the Principal Investigator, and the name and phone number of the person to contact. The “Principal Investigator” (also called the Site Investigator) is the physician who is heading up the study at that location. The contact person is typically the Research Coordinator, who is often a nurse or administrative assistant trained to coordinate clinical trials.
The Research Coordinator takes responsibility for many of the hands-on and day-to-day tasks in conducting the trial, including patient registration, data collection, safety monitoring, and coordination with the site investigator and principal investigator.